Abstract
Overexpression of the MYC oncogene is a frequent feature of diffuse large B-cell lymphoma (DLBCL) being associated with poor prognosis following standard R-CHOP (Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, Prednisone) chemoimmunotherapy. Since MYC expression is associated with overactivation of the DNA damage response (DDR), targeting DDR pathways with selective small molecule inhibitors could be a promising strategy to circumvent the inherent resistance to exogenous DNA damage proper of MYC-positive DLBCL. Loncastuximab tesirine-lpyl (here abbreviated as Lonca) is an antibody-drug conjugate (ADC) composed of a humanized anti-CD19 antibody conjugated to a potent DNA-crosslinking pyrrolobenzodiazepine dimer toxin.
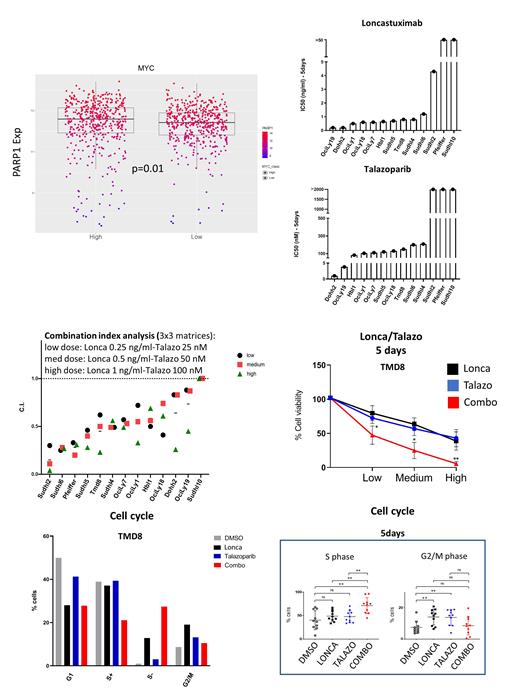
We hypothesized that DDR inhibition could increase the efficacy of Lonca by selectively enhancing DNA damage induction in B-cell lymphoma cells. In a preliminary analysis of a publicly available dataset (Sha et al. J Clin Oncol 2019), we found a significant correlation between MYC and PARP1 gene expression levels, with higher PARP1 levels observed in DLBCL samples characterized by high MYC expression. Following this observation, with the aim of developing a treatment strategy for MYC-positive DLBCL, we tested the in vitro activity of 3 FDA-approved PARP inhibitors (Olaparib, Rucaparib, Talazoparib) in a panel of 13 CD19+ DLBCL cell lines (7 with MYC rearrangements). Cytotoxicity was evaluated with Cell Titer Glo assay (CTG), and all compounds showed anti-proliferative activity in a dose and time-dependent manner. Compared with Olaparib and Rucaparib, Talazoparib (Talazo) showed more potent in vitro activity as single agent, with IC50 values in the submicromolar range (100-200 nM at 5 days) observed in most cell lines, in line with its increased PARP trapping capacity. Of note, the BRCA-mutated cell line DOHH2 was the most sensitive to all 3 PARPi, in line with the known synthetic lethal interaction between BRCA mutations and PARP inhibition.
Lonca showed significant cytotoxic activity in 10 of 13 cell lines, which were sensitive in the same range of clinically achievable concentrations, with IC50 values ≤ 1 ng/ml at 5 days; conversely, the B12 isotype control ADC was completely ineffective. Interestingly, BRCA-mutated DOHH2 cells were highly sensitive to single agent Lonca, and those cell lines that were resistant to Lonca (SUDHL-2, Pfeiffer, SUDHL-10) displayed also reduced sensitivity to Talazo. The combination of Talazo and Lonca produced enhanced anti-proliferative effects, with strong synergism (evaluated by combination index analysis) observed in most cell lines. Notably, those cell lines resistant to both compounds as single agents became sensitive to the combination. To investigate the mechanisms underlying these synergistic interactions, we performed 2D-cell cycle analysis by flow cytometry with bromodeoxyuridine (BrdU) and propidium iodide staining at different time points (24h-72h-5days). While single agent treatments predominantly increased the fraction of cells in G2/M phase, the combination of Talazo and Lonca clearly increased the fraction of cells in the BrdU-negative S phase of the cell cycle, (which represents cells that are not actively synthetizing DNA, S-), thus suggesting a mechanistic interaction between the two drugs (Fig. 1). Treatment with the Lonca/Talazo combination induced cell death (as measured by trypan blue assay or SubG1 fraction of the cell cycle), in the absence of significant caspase cleavage. Western blot assay showed enhanced induction of the DNA damage marker γH2AX in cells treated with the combination after 24 hours of incubation. In line with this, alkaline Comet assay confirmed enhanced DNA damage induction after 24h of combinational treatment as compared to single agents, thus confirming that DDR inhibition significantly increases the amount of DNA damage induced by Lonca. Comet assay performed on peripheral blood CD19+ B cells from healthy donors following ex-vivo treatment with Talazo, Lonca or the combination showed similar DNA damage induction patterns. Importantly, no DNA damage could be detected in CD3+ T-cells from the same healthy donors.
In conclusion, these data indicate that PARP inhibition significantly enhances the efficacy of Lonca in in vitro DLBCL models, providing the rationale to develop precision therapy strategies aimed at boosting DNA damage induction in neoplastic B-cells.
Zammarchi: ADC Therapeutics: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. van Berkel: ADC Therapeutics: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Pileri: NANOSTRING: Other: ADVISORY BOARD; ROCHE: Other: ADVISORY-BOARD; CELGENE: Other: ADVISORY BOARD. Tarella: ADC-THERAPEUTICS: Other: ADVISORY BOARD; Abbvie: Other: ADVISORY BOARD. Derenzini: TG-THERAPEUTICS: Research Funding; ASTRA-ZENECA: Consultancy, Other: ADVISORY-BOARD; BEIGENE: Other: ADVISORY BOARD; TAKEDA: Research Funding; ADC-THERAPEUTICS: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal